One-Year Follow-Up: DESS® Implants Match Premium Brands in Marginal Bone Stability

By Dr. Mohamed Khalifa, BDS, MSc, MSc (Clin) – DESS® KOL, Saudi Arabia
Introduction
Following the success of my initial 3-month study published in 2024, now I’m presenting a one-year clinical follow-up comparing DESS® implants with three internationally recognized premium systems — Straumann® (SLA), Astra Tech™ (OsseoSpeed) and Nobel Biocare® (TiUnite).
The first study demonstrated no statistically significant differences in marginal bone loss after 3 months, establishing DESS® as a clinically equivalent, high-performance option for predictable osseointegration.
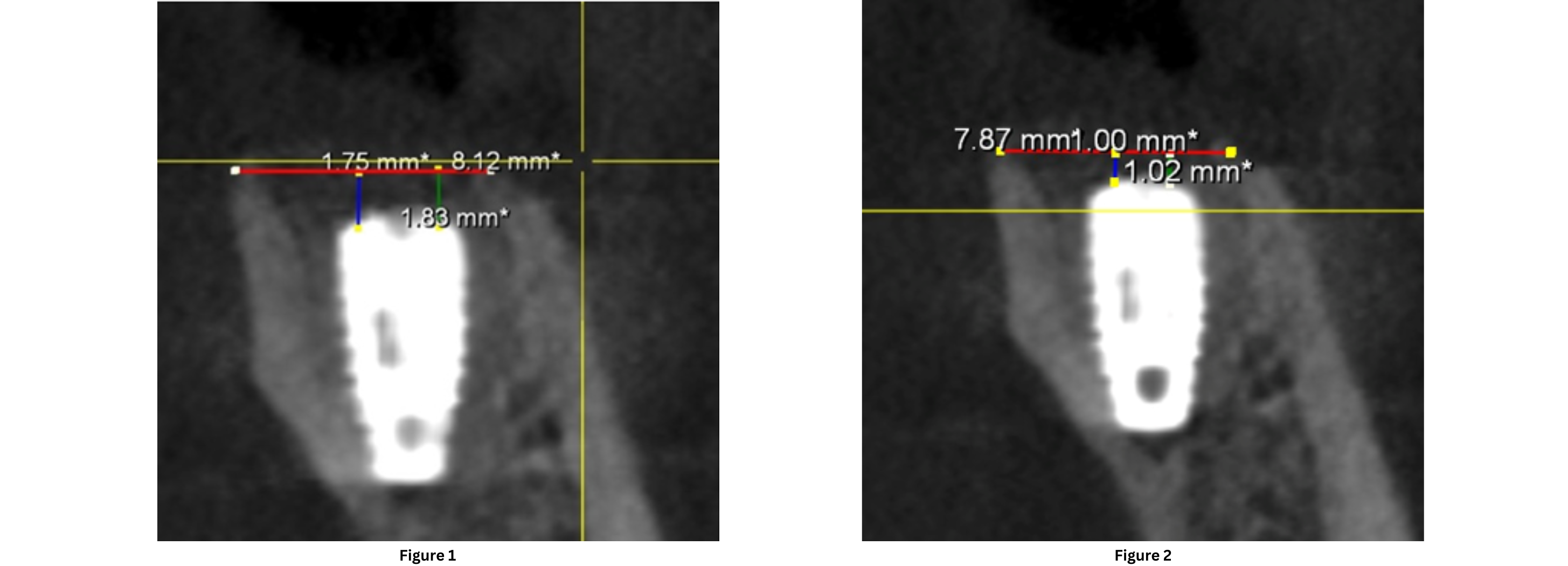

Figure 1: This CBCT image was taken immediately after implant placement. It shows the initial condition of the crestal bone level.
Figure 2: This CBCT image was taken 3 months after implant placement. It shows the crestal bone level measurement after 3 months.
Study Design and Methodology
The one-year follow-up continued with the same non-randomised prospective cohort of 40 patients, each receiving one mandibular implant (10 per system).
CBCT scans were performed at baseline, 3, 6, and 12 months to assess marginal bone changes.
Two calibrated examiners conducted all measurements, achieving ICC = 0.95, indicating excellent inter-examiner reliability.
Clinical Findings
After 12 months, all four implant systems maintained healthy crestal bone levels and stable soft tissue.
Although Straumann® and Astra Tech® presented slightly lower mean bone-loss values, the difference of 0.02–0.04 mm was clinically negligible.
The DESS® CONICAL BLT Implant, featuring the Osseointegration Surface Technology (OST by DESS®) — a dual acid-etched, alumina-blasted surface developed to SLA®-level standards — achieved the same biological performance and stability as the premium implant systems.
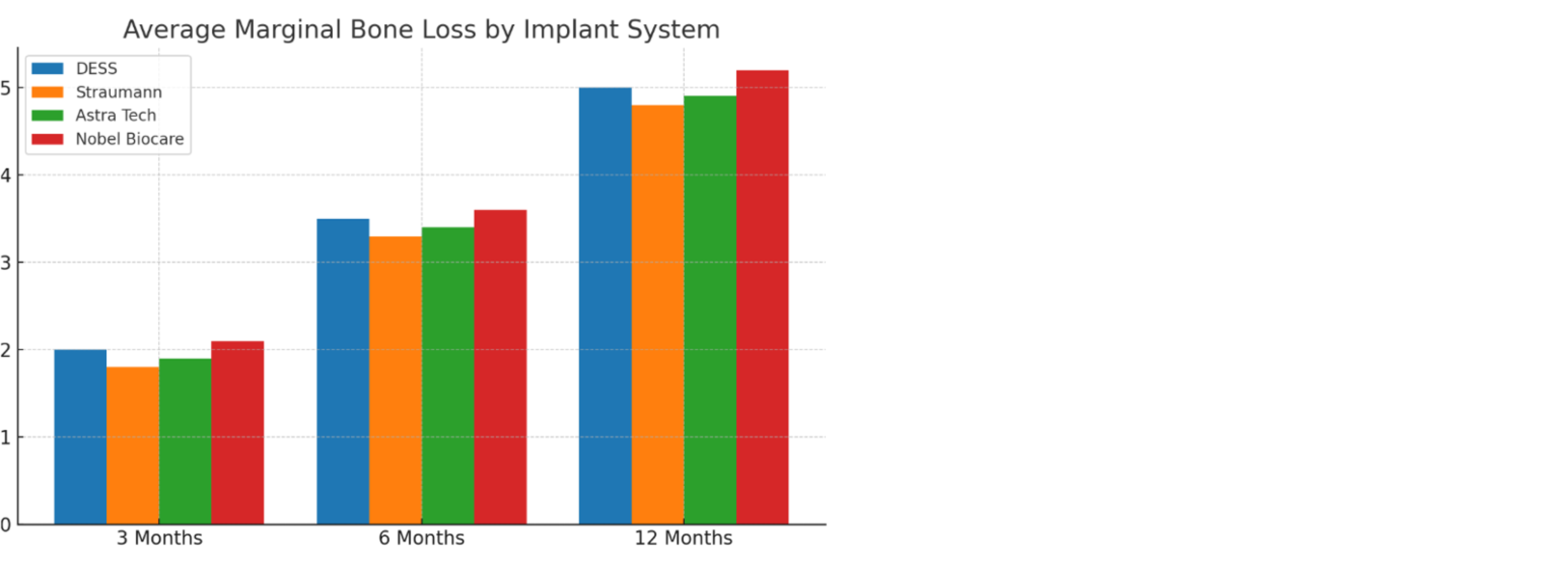

Table 1: Average Marginal Bone Loss Over Time.
Key Takeaways
- Comparable bone preservation: All systems remained within a 0.04 mm difference in one year.
- Healthy peri-implant tissue: Stable soft-tissue profiles were observed across all groups.
- Clinical reliability: DESS® demonstrates equivalent biological performance to premium implants.
- Cost efficiency: Comparable outcomes at a significantly more accessible price point.
- Digital-ready: Fully compatible with modern digital workflows and restorative solutions.
Clinical Implications
With no statistically significant differences in bone stability between systems, implant selection can confidently be guided by clinical flexibility, prosthetic compatibility, and cost-efficiency rather than bone-loss concerns in the first year.


Figure 3: CBCT taken 1 year follow-up.
Conclusion
This one-year clinical follow-up confirms that DESS® CONICAL BLT Implants provide marginal bone stability equivalent to leading premium systems — Straumann®, Astra Tech®, and Nobel Biocare®.
|
Implant System |
3 Months |
6 Months |
12 Months |
|
DESS® |
0.2 |
0.35 |
0.5 |
|
Straumann® |
0.18 |
0.33 |
0.48 |
|
Astra Tech™ |
0.19 |
0.34 |
0.49 |
|
Nobel Biocare® |
0.21 |
0.36 |
0.52 |
Table 2: Results Average Marginal Bone Loss (mm).
These findings reinforce DESS®’s position as a clinically validated and cost-efficient option for clinicians seeking predictable, high-quality results in everyday implantology.
|
Criteria |
Description |
DESS® Implants |
|
Compatibility |
Offers diverse designs, various cases. |
✔ |
|
Ease of Use |
Provides user-friendly surgical kits, training, and ongoing support. |
✔ |
|
Aesthetic Focus |
Promotes healthy soft tissue and offers multiple aesthetic solutions. |
✔ |
|
Cost vs. Quality |
Balances high quality and long-term value with affordability. |
✔ |
|
Warranty & Availability |
Ensures reliable warranties. |
✔ |
|
Futureproofing |
Solution compatible with digital workflows. |
✔ |
Table 3.